New paper from Maria (PhD thesis, paper No.2)
2018-01-01
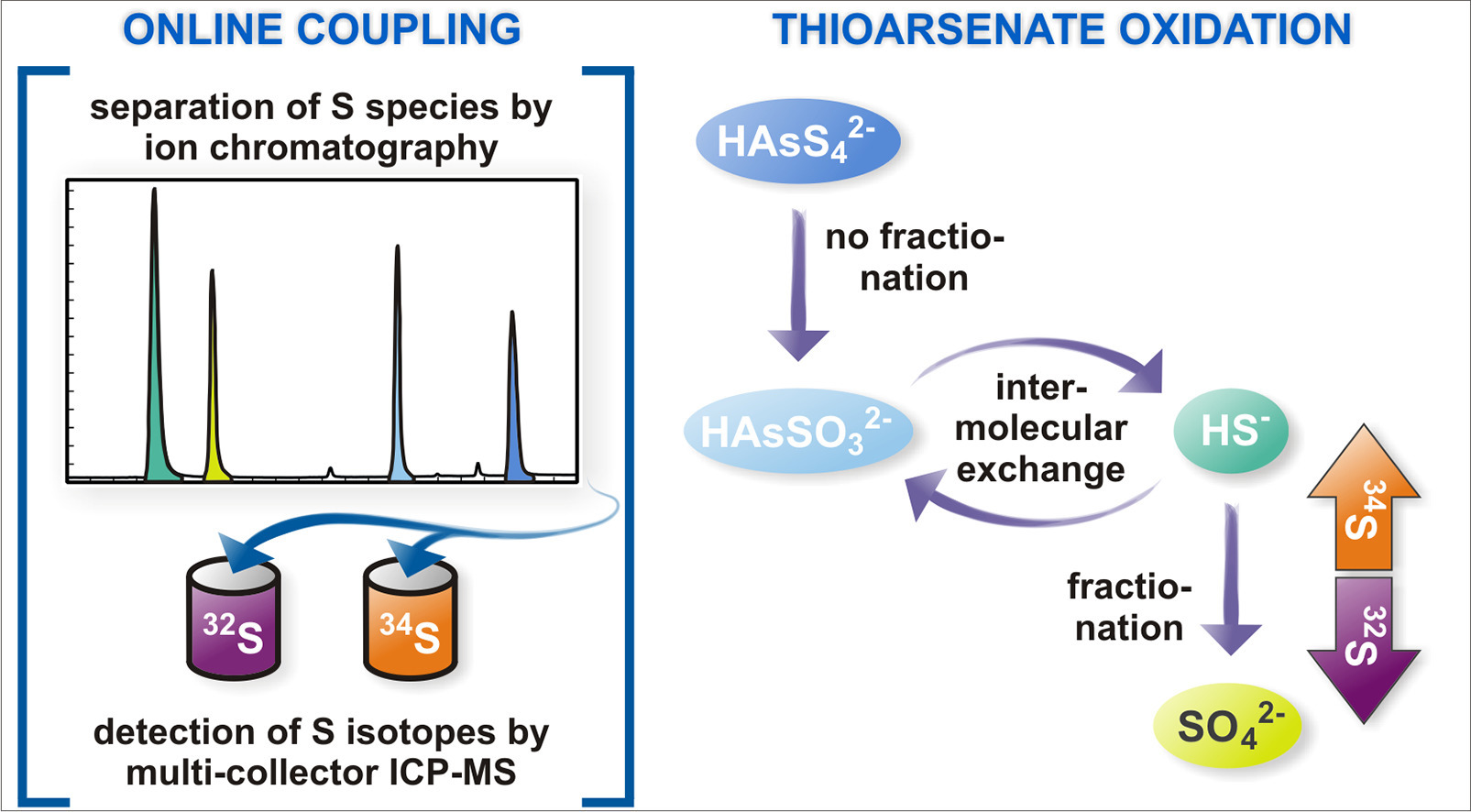
As part of her PhD thesis, Maria Ullrich published a new paper on "Sulfur isotope analysis by IC-MC-ICP-MS provides insight into fractionation of thioarsenates during abiotic oxidation" in Chemical Geology, 477, 92-99 (2018) doi:10.1016/j.chemgeo.2017.12.008
Abstract. Standard sulfur isotope analysis of aqueous samples is aimed at sulfide and sulfate, while often disregarding intermediate species, mainly due to analytical limitations. However, sulfur can form numerous intermediate species in the environment, including thiometal(loid)s such as thioarsenates ([HAsVS− IInO4 − n]2 −, n = 1–4), which can play a key role in isotope fractionation. The standard precipitation procedure for separation of sulfide and sulfate was applied to monothioarsenate solutions. Monothioarsenate was found to co-precipitate together with sulfide, thus potentially impeding correct sulfide isotope analysis. To overcome the limitations associated with this standard precipitation procedure, a new method was developed based on separating sulfur species by ion chromatography followed by online isotope detection on multi-collector ICP-MS (IC-MC-ICP-MS). Applying this new method, fractionation between monothioarsenate and sulfate of up to − 6.1‰ was found during monothioarsenate oxidation. In contrast, oxidation of tetrathioarsenate via tri- and di- to monothioarsenate did not result in fractionation. However, the released sulfide became increasingly enriched in 34S due to oxidation to sulfate. This process was found to introduce additional enrichment of up to 9.1‰ to monothioarsenate through intermolecular isotope exchange between arsenic-bound sulfur and sulfide in solution. These results help elucidate pathways of thioarsenate transformation, and thus provide valuable information for the interpretation of isotope fractionation patterns in sulfidic environments.

