New paper from Carolin (MSc thesis)
02.02.2017
From her MSc thesis completed in cooperation with Prof. Derek Vance, Institute for Geochemistry and Petrology, ETH Zürich, Carolin Kerl published a new paper on "Experimental Confirmation of Isotope Fractionation in Thiomolybdates Using Ion Chromatographic Separation and Detection by Multicollector ICPMS” in Analytical Chemistry
Kerl, C; Lohmayer, R; Bura-Nakic, E; Vance, D; Planer-Friedrich, B: Experimental confirmation of isotope fractionation in thiomolybdates using ion chromatographic separation and detection by multi-collector ICP-MS, Analytical Chemistry, 89(5), 3123–3129 (2017), doi:10.1021/acs.analchem.6b04898
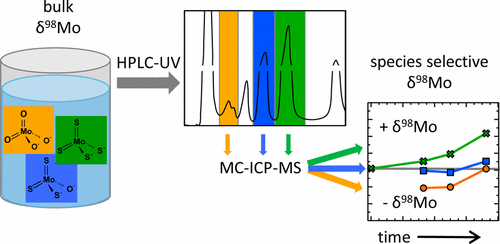
Abstract. Molybdenum 98Mo/95Mo isotope ratios are a sediment paleo proxy for the redox state of the ancient ocean. Under sulfidic conditions, no fractionation between seawater and sediment should be observed if molybdate (MoO42–) is quantitatively transformed to tetrathiomolybdate (MoS42–) and precipitated. However, quantum mechanical calculations previously suggested that incomplete sulfidation could be associated with substantial fractionation. To experimentally confirm isotope fractionation in thiomolybdates, a new approach for determination of isotope ratios of individual thiomolybdate species was developed that uses chromatography (HPLC-UV) to separate individual thiomolybdates, collecting each peak and analyzing isotope ratios with multicollector inductively coupled plasma mass spectrometry (MC-ICPMS). Using commercially available MoO42– and MoS42– standards, the method was evaluated and excellent reproducibility and accuracy were obtained. For species with longer retention times, complete chromatographic peaks had to be collected to avoid isotope fractionation within peaks. Isotope fractionation during formation of thiomolybdates could be experimentally proven for the first time in the reaction of MoO42– with 20-fold or 50-fold excess of sulfide. The previously calculated isotope fractionation for MoS42– was confirmed, and the result for MoO2S22– was in the predicted range. Isotopic fractionation during MoS42– transformation with pressurized air was dominated by kinetic fractionation. Further optimization and online-coupling of the HPLC-MC-ICPMS approach for determination of low concentrations in natural samples will greatly help to obtain more accurate species-selective isotope information.

